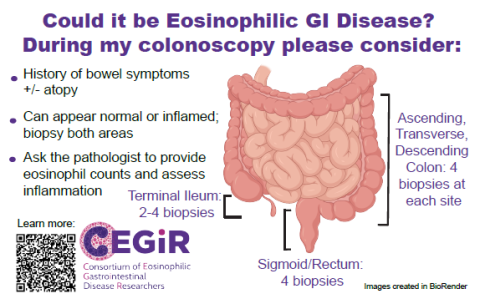
Endoscopy Best Practices
Welcome patients, providers, and caregivers! As eosinophilic GI disease (EGID) researchers and patient advocates, we know that obtaining an EGID diagnosis and keeping up to date with the rapidly changing research can be challenging. The goal of this page is to be a resource for the most up to date information regarding optimal testing for and diagnosis of EGIDs.
The Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) is an NIH-funded consortium that connects EGID researchers, Patient Advocacy Groups, and Patients to perform and participate in research and advocacy for eosinophilic GI disease. Diagnostic requirements and criteria including:
- What we know about the “typical” appearance of a GI tract affected by EGID
- Number of biopsies needed from each portion of the GI tract to diagnose EGID
- The number of eosinophils per high power field on pathology consistent with EGID are frequently changing in these rare diseases that are often associated with significant diagnostic delay.
As a collaboration between the physicians and patient advocates within CEGIR, we have created this reference card and website as a “quick cheat sheet” guide to diagnostic recommendations for EGIDs.
Symptoms/Why think about EGID?
Because the clinical presentation of EGIDs can vary widely, patients and providers need to have a high index of suspicion on when to consider an EGID diagnosis. Symptoms tend to be non-specific, and include nausea, vomiting, abdominal and/or chest pain, diarrhea, bloating, weight loss, poor growth, feeding difficulties, difficult swallowing, food impactions, fatigue and early satiety. Non-specific associations along with an associated peripheral eosinophilia and/or an atopic history including eczema, asthma, rhinitis, or food allergy or intolerance should raise the suspicion of an EGID even more. In addition, symptoms often vary depending on which part of the GI tract is involved. As an example, patients with eosinophilic esophagitis (EoE) most often present with trouble swallowing or dysphagia, whereas a patient with eosinophilic gastritis (EoG) may present more with nausea, vomiting and early satiety. Furthermore, a patient with eosinophilic colitis (EoC) can often present with chronic diarrhea, bloody diarrhea or weight loss. While there are classic presentations of EGIDs, symptoms remain largely non-specific and may progress to many different complications as the initial presentation. Complications such as peripheral edema, gastrointestinal bleeding, ulcers, strictures, intestinal obstruction and unexplained anemia raise the suspicion for non-EoE EGIDs. To avoid these complications, prompt and accurate diagnosis and detection is helpful.
When relatively common non-specific symptoms persist or these complications arise without a clear reason, EGIDs should be considered in the differential diagnosis. To aid the diagnosis, endoscopic tissue biopsies from multiple areas of the gastrointestinal tract provide essential information when considering these diagnoses. Importantly, endoscopy findings often grossly look visually normal as these diseases tend to be very patchy. Because of the patchy nature, it is essential to look at the microscopic level with an adequate number of biopsies when strongly considering these diagnoses. Once biopsies are obtained, it is recommended to discuss the clinical concerns of EGIDs with the pathologist examining the biopsies to have a thorough evaluation.
Approach to Diagnosis
Much of our understanding of EGIDs is rooted and extrapolated from EoE, however growing research supports the above reference values for eosinophils as cut off values particularly when eosinophil counts are seen with other signs of mucosal injury. Endoscopic evaluation with tissue biopsies is required for the diagnosis for EoE and non-EoE EGIDS, regardless if the mucosa looks normal as disease can be patchy. Due to the patchy nature of these diseases, multiple biopsies are needed to maximize the diagnostic sensitivity of EGIDs. Ongoing studies have identified at least six biopsies from in the esophagus, eight biopsies from the stomach, and four biopsies from the duodenum for good sensitivity, and that biopsies should be taken from both normal and abnormal appearing mucosa. Additional work is needed to establish the ideal number of biopsies needed from the distinct areas of the colon.
Future Directions and What’s on the Horizon
EGID diagnosis, management and understanding is rapidly expanding and evolving, we have a few directions to highlight for anticipated updates to the field (and website).
In 2023, international guidelines were released for pediatric EGIDs and can be found here. In these guidelines, recommendations for the diagnosis and evaluation of pediatric EGID were made. Some of the major highlights include symptomatic and histologic guidance seen in the graphical abstract below.
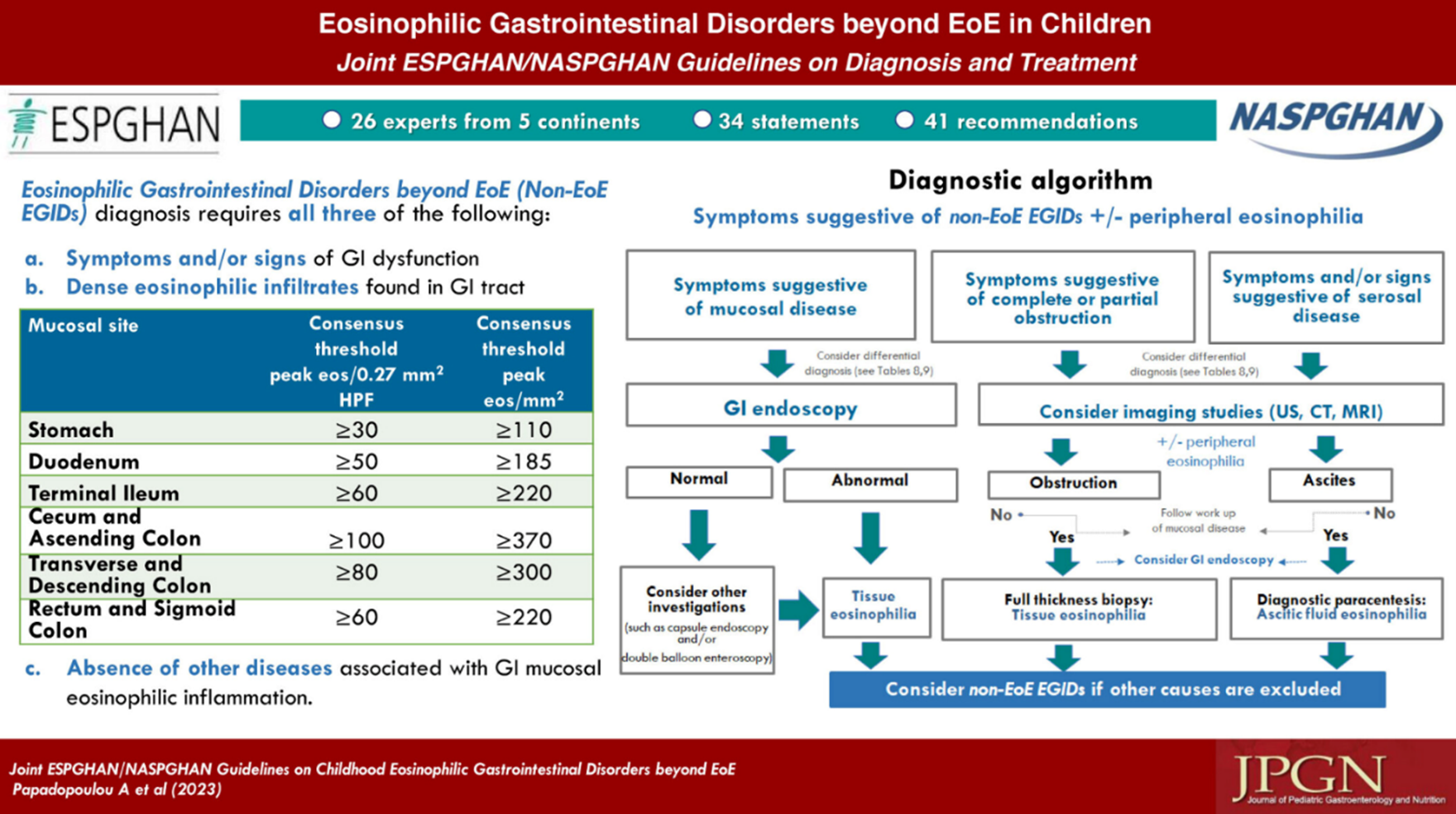
With much anticipation, the EGID field awaits clinical and histologic guidance for adults which will be announced in the near future. Check back here for pertinent updates.
For the time being, CEGIR recommends obtaining biopsies at the time of endoscopy in patients with a history of allergy or clinical pictures suggestive of EGID. We recommend prompting the pathologist with a possible diagnosis to evaluate for EGID. The general histologic consensus currently is largely based on pediatric guidelines with their values shown above, but these may change in the future and as adult data is synthesized. We recognize this can be frustrating and the field is making every effort to streamline and provide accurate information.
References:
- Shah A, Kagalwalla AF, Gonsalves N, Melin-Aldana H, Li BU, Hirano I. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol. 2009;104(3):716-721. doi:10.1038/ajg.2008.117
- Nielsen JA, Lager DJ, Lewin M, Rendon G, Roberts CA. The optimal number of biopsy fragments to establish a morphologic diagnosis of eosinophilic esophagitis. Am J Gastroenterol. 2014;109(4):515-520. doi:10.1038/ajg.2013.463
- Wechsler JB, Bolton SM, Gray E, Kim KY, Kagalwalla AF. Defining the Patchy Landscape of Esophageal Eosinophilia in Children With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2022;20(9):1971-1976.e2. doi:10.1016/j.cgh.2021.12.023
- Papadopoulou A, Amil-Dias J, Auth MK, et al. Joint ESPGHAN/NASPGHAN Guidelines on Childhood Eosinophilic Gastrointestinal Disorders Beyond Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2024;78(1):122-152. doi:10.1097/MPG.0000000000003877
- Dellon ES, Gonsalves N, Rothenberg ME, Hirano I, Chehade M, Peterson KA, Falk GW, Murray JA, Gehman LT, Chang AT, Singh B, Rasmussen HS, Genta RM. Determination of Biopsy Yield That Optimally Detects Eosinophilic Gastritis and/or Duodenitis in a Randomized Trial of Lirentelimab. Clin Gastroenterol Hepatol. 2022 Mar;20(3):535-545.e15. doi: 10.1016/j.cgh.2021.05.053. Epub 2021 Jun 2. PMID: 34089846; PMCID: PMC8636525